Mesoporous palladium—the surface electrochemistry of palladium in aqueous sodium hydroxide and the cathodic reduction of nitrite
Abstract
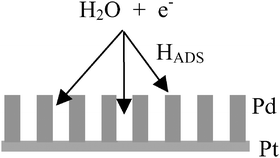
The voltammetry of nanostructured palladium layers electrodeposited from a hexagonal liquid crystal phase onto platinum microdiscs show well defined peaks for the adsorption/desorption of hydrogen and surface oxidation/reduction in 2 M NaOH. These peaks are more clearly resolved than at smooth palladium and reveal the complications associated with hydrogen adsorption/desorption on palladium in aqueous alkaline solutions. The reduction of nitrite at the nanostructured palladium is also reported and it is shown that it occurs via a mechanism involving a chemical reaction between adsorbed hydrogen and adsorbed nitrite ion.

 Please wait while we load your content...
Please wait while we load your content...