Indices for predicting the quality of leaving groups
Abstract
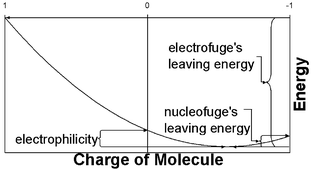
The inherent quality of leaving groups in chemical reactions is related to their ionization potential and electron affinity using a quadratic model for the dependence of the energy on the number of electrons. A good leaving group for nucleophilic substitution/elimination reactions is one where the difference in energy between the system with the “optimum” number of electrons and the anion is small. Similarly, a good leaving group for electrophilic substitution/elimination reactions is one where the difference in energy between the system with the optimum number of electrons and the

 Please wait while we load your content...
Please wait while we load your content...