Gold–polyaniline composite
Part I. Moving electrochemical interface†
Abstract
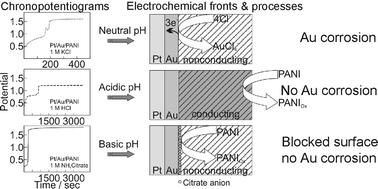
The controlled growth of gold nanoclusters in polyaniline is predicated by the ability of PANI to switch from conducting to insulating form and by the fact that

 Please wait while we load your content...
Please wait while we load your content...