Kinetics of the gas phase HO2 self-reaction: Effects of temperature, pressure, water and methanol vapours†
Abstract
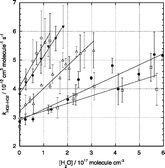
The kinetics of the gas phase HO2 self-reaction have been studied using flash photolysis of Cl2/CH3OH/O2/N2 mixtures coupled with time-resolved broadband UV absorption spectroscopy. The HO2 self-reaction rate coefficient (HO2 + HO2 → H2O2 + O2 (R1)) has been determined as a function of temperature (236 < T < 309 K, at 760 Torr) and pressure (100 < p < 760 Torr, at 296 K). In addition, the effects of water vapour ((0–6.0) × 1017 molecules cm−3, 254 < T < 309 K at 760 Torr, 400 < p < 760 Torr at 296 K) and methanol vapour ((0.06–4.7) × 1017 molecules cm−3, 254 < T < 309 K, at 760 Torr) on the rate coefficient have been characterised. The observed rate coefficient, k1, was found to exhibit a negative temperature dependence with both pressure dependent and pressure independent components, in agreement with previous studies. Furthermore, the rate coefficient k1 was found to be enhanced in the presence of elevated H2O or CH3OH concentrations, as reported previously. This study reports the most extensive characterisation of the rate coefficient k1 as a function of T, p, [H2O] and [CH3OH]. The present results indicate that k1 is higher at low temperatures, and that enhancement of k1 by H2O is greater, than has been reported previously. The pressure dependence of k1 at ambient temperature is in good agreement with previous studies. The rate enhancement by CH3OH reported here is in good agreement with previous studies at ambient temperatures but is smaller at low temperatures than the most recent previous investigation suggests. The rate coefficient k1 is adequately parameterised by: k1(760 Torr) = {(1.8 ± 0.8) × 10−14 exp((1500 ± 120)/T/K)} × {1 + (2.0 ± 4.9) × 10−25 [H2O] exp((4670 ± 690)/T/K)} × {1 + (0.56 ± 1.00) × 10−21 [CH3OH] exp((2550 ± 500)/T/K)} cm−3 molecule−1 s−1, where [H2O] and [CH3OH] are in molecules cm−3. Errors are 1σ, and statistical only. The atmospheric implications of these results are briefly discussed.

 Please wait while we load your content...
Please wait while we load your content...