Theoretical investigation of the absorption and ionization spectrum of the super greenhouse gas SF5CF3
Abstract
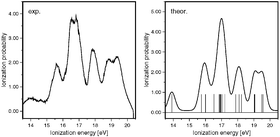
SF5CF3, recently found in the Earth’s atmosphere, is an extremely potent greenhouse gas. Its behaviour under irradiation, as in the upper atmosphere, is of great importance for the possible impact on the global climate. The vertical

 Please wait while we load your content...
Please wait while we load your content...