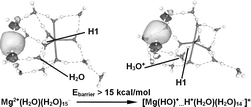
Mg+(H2O)n clusters ions are known to undergo a hydrogen loss reaction to produce (MgOH)+(H2O)n−1, when the cluster size n falls between 5 and 15. It indicates that the microscopic solvation environment has a strong effect on the reaction mechanisms, which have now been elucidated. Above n
= 6, Mg+(H2O)n exists in the form of an ion pair, Mg2+(H2O)l⋯e−(H2O)n−l, and H+ is produced by the autoionization of H2O, enhanced by the hydrolysis of Mg2+. Production of the H atom is due to a reductive half reaction H+
+ e → H, with the electron being the solvated electron in the ion pair. The reaction barrier is reduced when the electron is in the first or second solvation shell, promoting the H loss reaction. As the number of H2O molecules increases, the electron moves beyond the third solvation shell and the reaction barrier increases significantly, which is responsible for the subsequent switch-off of H loss. It has been one of the important goals in cluster sciences to understand the microscopic correlation between solvation and chemical reactivity by in-depth study on clusters, which has now been achieved in the case of Mg+(H2O)n, linking electron solvation with reaction paths for electron transfer.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...