Ion–molecule kinetics at 15–700 Torr†
Abstract
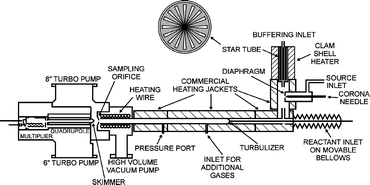
Studies of ion–molecule chemistry are usually made at pressures of a few Torr and below. By contrast, there are numerous plasmas that occur at higher pressures. For that reason we have constructed a turbulent ion flow tube (TIFT) for studying ion–molecule kinetics from 15 to 700 Torr. Currently, the TIFT operates from room temperature to 700 K. Here we present a summary of the measurements we have made to date. The first measurements involved SF6− reactions with SO2, H2O, CH3OH and C2H5OH at room temperature. The SO2 reaction showed the same kinetics as low pressure measurements indicating that the reaction occurs rapidly. The other reactions were all found to be cluster-mediated with branching fractions that depend on pressure. More recently, charge transfer reactions of O2+ to alkylbenzenes have been studied at elevated temperatures, from 400 to 700 K. Both dissociative and non-dissociative charge transfer occurs with the latter being favored at high pressures indicating that excited states live long enough to be stabilized by the buffer gas. Combining the TIFT measurements with detailed statistical adiabatic channel model/classical trajectory (SACM/CT) calculations of the unimolecular decay constant allows energy transfer parameters to be derived. Extending the temperature range upwards to 750 K has allowed thermal decomposition rate constants to be measured. The thermal decomposition has been successfully modeled using the same parameters as for the collision quenching modeling. This allows bond strengths for the dissociation to be derived with high accuracy. Both the measurements and models show that the conditions correspond to the high pressure kinetics regime.

 Please wait while we load your content...
Please wait while we load your content...