The covalent, zwitterionic and biradical forms of the MCl(H2O)4
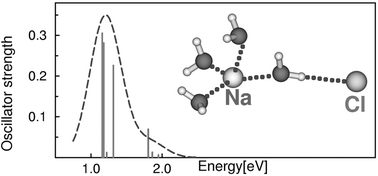
(M = H, Li, Na) clusters have been investigated with density functional theory (DFT), time-dependent DFT (TDDFT) and the second-order Møller–Plesset (MP2) method. The equilibrium geometries and force fields of the lowest electronic states have been determined with DFT and MP2; vertical electronic excitation energies have been calculated with TDDFT at the DFT geometries. It is shown that the excited states of the M+(H2O)4Cl− zwitterions are of the charge-transfer-to-solvent (CTTS) type. The molecular and electronic structures of the M(H2O)4Cl biradicals (M = Li, Na) have been characterized for the first time. The lowest electronic states of the biradicals are lower in energy than the CTTS excited states of the zwitterions, and therefore are photochemically accessible from the latter. The electronic absorption spectra of the biradicals are essentially identical with that of the hydrated hydronium radical, H3O(H2O)3, and exhibit striking similarities with the spectral signatures of the hydrated electron in the liquid phase. It is argued that the photochemistry of the M+(H2O)3Cl− zwitterions represents a finite-size molecular model of the formation process of the hydrated electron via the photodetachment of the chloride anion in concentrated salt solutions.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...