Hydrogen formation in the reaction of Zn+(H2O)n with HCl
Abstract
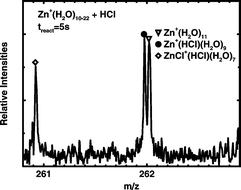
Hydrated singly charged zinc cations Zn+(H2O)n, n ≈ 6–53, were studied by Fourier transform ion cyclotron resonance (FT-ICR) mass spectrometry. Black-body radiation induced dissociation results exclusively in sequential loss of individual water molecules. In the reaction of Zn+(H2O)n with gaseous HCl, Zn is oxidized and hydrogen reduced when a second HCl molecule is taken up, leading to the formation of ZnCl+(HCl)(H2O)n−m cluster ions and evaporation of atomic hydrogen together with m H2O molecules. The results are compared with earlier studies of Mg+(H2O)n, for which hydrogen formation is already observed without HCl in a characteristic size region. The difference between zinc and magnesium is rationalized with the help of density functional theory calculations, which indicate a distinct difference in the thermochemistry of the reactions involved. The generally accepted hydrated electron model for hydrogen formation in Mg+(H2O)n is modified for zinc to account for the different reactivity.

 Please wait while we load your content...
Please wait while we load your content...