Palladium-catalyzed, stereoselective rearrangement of a tetracyclic allyl cyclopropane under arylation†
Abstract
The first example of a π,σ domino-Heck reaction under concomitant rearrangement of the tetracyclic allylcyclopropane endo,exo-bishomobarrelene (5) is reported; the stereoselective reaction proceeds via an intramolecular insertion of a primarily-formed carbopalladation intermediate into a strained
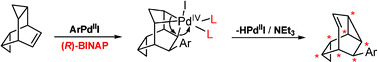

 Please wait while we load your content...
Please wait while we load your content...