Reversible caterpillar-motion like isomerization in a N,N′-dimethyl hexaphyrin(1.1.1.1.1.1) induced by two-electron oxidation or reduction†
Abstract
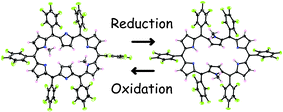
Caterpillar-motion like rotational isomerization of meso-aryl substituted [26]- and [28]hexaphyrins has been revealed for the first time. Two-electron oxidation and reduction induce reversible interconversion between N,N′-dimethyl [26]- and [28]hexaphyrins with a rotational isomerization, in which the N-methylated pyrroles move from the corner in the former to the centre in the latter.

 Please wait while we load your content...
Please wait while we load your content...