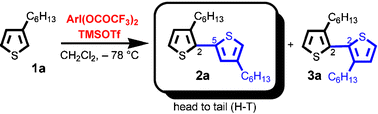
The synthesis of head-to-tail (H–T) dimers of 3-substituted thiophenes by the hypervalent iodine(iii)-induced oxidative biaryl coupling reaction
Abstract
The head-to-tail (H–T) dimers could be obtained selectively by the oxidative coupling reaction of 3-substituted

 Please wait while we load your content...
Please wait while we load your content...