Stereochemistry of the reaction catalysed by 2-hydroxy-6-keto-6-phenyl-hexa-2,4-dienoic acid 5,6-hydrolase (BphD)
Abstract
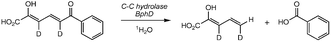
The stereochemical course of the reaction catalysed by C-C hydrolase BphD from Burkholderia xenovorans LB400 occurs with replacement of a

 Please wait while we load your content...
Please wait while we load your content...