Highly enantioselective dynamic kinetic resolution and desymmetrization processes by cyclocondensation of chiral aminoalcohols with racemic or prochiral δ-oxoacid derivatives†
Abstract
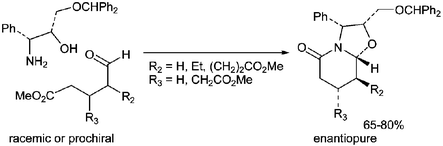
Cyclocondensation reactions of aminoalcohols 7 and 8 with racemic or prochiral δ-oxoacid derivatives provide polysubstituted

 Please wait while we load your content...
Please wait while we load your content...