Analytical chemistry of synthetic routes to psychoactive tryptamines
Part III.† Characterisation of the Speeter and Anthony route to N,N-dialkylated tryptamines using CI-IT-MS-MS
Abstract
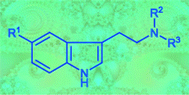
Twelve symmetrically and 13 asymmetrically N,N-disubstituted ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N+R2R3]
N+R2R3]

 Please wait while we load your content...
Please wait while we load your content...