Synthesis and stability of small molecule probes for Pseudomonas aeruginosa quorum sensing modulation†
Abstract
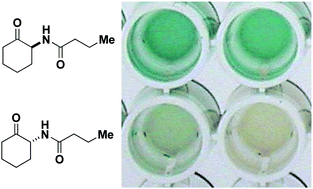
The human pathogen Pseudomonas aeruginosa uses N-butyryl-L-homoserine lactone (BHL) and N-(3-oxododecanyl)-L-homoserine lactone (OdDHL) as small molecule intercellular signals in a phenomenon known as quorum sensing (QS). QS modulators are effective at attenuating P. aeruginosa virulence; therefore, they are a potential new class of

 Please wait while we load your content...
Please wait while we load your content...