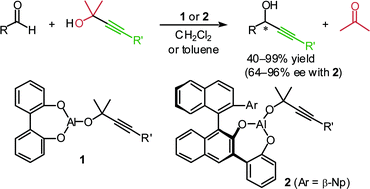
A novel carbonyl alkynylation has been accomplished based on utilization of the Meerwein–Ponndorf–Verley (MPV) reaction system. The success of the MPV alkynylation crucially depends on the discovery of the remarkable ligand acceleration effect of 2,2′-biphenol. For example, the alkynylation of chloral (2c) with the aluminium alkoxide 6
(R = Ph), prepared in situ from Me3Al, 2,2′-biphenol and 2-methyl-4-phenyl-3-butyn-2-ol (1a) as an alkynyl source, proceeded smoothly in CH2Cl2 at room temperature to give the desired propargyl alcohol 3ca in almost quantitative yield after 5 h stirring. The characteristic feature of this new transformation involving no metal alkynides can be visualized by the fact that the alkynyl group bearing keto carbonyl was transferred successfully to aldehyde carbonyl without any side reactions on keto carbonyl. Although the use of (S)-1,1′-bi-2-naphthol and its simple analogues was found to be unsuitable for inducing asymmetry in this reaction, design of new chiral biphenols bearing a certain flexibility of the biphenyl axis led to satisfactory results in terms of enantioselectivity as well as reactivity.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...