Probing the effect of the amidinium group and the phenyl ring on the thermodynamics of binding of benzamidinium chloride to trypsin
Abstract
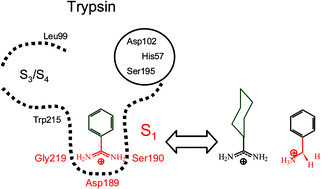
The effect of the amidinium group and the phenyl ring on the thermodynamics of binding of benzamidinium chloride to the serine proteinase trypsin has been studied using isothermal titration calorimetry. Binding studies with benzylammonium chloride, α-methylbenzylammonium chloride and benzamide, compounds structurally related to benzamidinium chloride, showed that hydrogen bonding between the amidinium group and the enzyme is primarily enthalpy-driven. Binding of cyclohexylcarboxamidinium chloride and acetamidinium chloride showed that the hydrophobic binding of the phenyl ring in the S1 pocket is primarily entropy-driven and that a rigid, flat hydrophobic binding site for the inhibitor is favourable. The compounds that have been studied over a range of temperatures exhibit a negative change in heat capacity upon binding and enthalpy–entropy compensation, both characteristic of hydrophobic interactions.

 Please wait while we load your content...
Please wait while we load your content...