Conjugate addition of organocopper reagents to γ-alkoxybutenolides and application to the synthesis of non-racemic alkyl cyclopentenones
Abstract
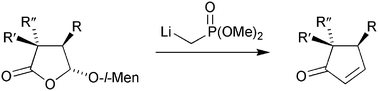
Simple organocopper reagents are shown to undergo anti-stereoselective 1,4-addition to menthyloxy-substituted lactone 1 in the presence of BF3·OEt2; the Lewis acid causes partial epimerisation of the acetal centre after conjugate addition. Enolate alkylation of the adducts leads to di- and trisubstituted lactones that are converted, in favourable cases, into di- and trisubstituted cyclopentenones.

 Please wait while we load your content...
Please wait while we load your content...