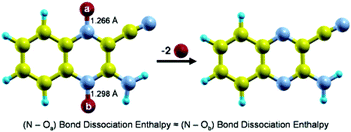
The standard enthalpy of formation of the 2-amino-3-quinoxalinecarbonitrile-1,4-dioxide compound in the gas-phase was derived from the enthalpies of combustion of the crystalline solid measured by static bomb combustion calorimetry and its enthalpy of sublimation determined by Knudsen mass-loss effusion at T
= 298.15 K. This value is (383.8 ± 5.4) kJ mol−1 and was subsequently combined with the experimental gas-phase enthalpy of formation of atomic oxygen and with the computed gas-phase enthalpy of formation of 2-amino-3-quinoxalinecarbonitrile, (382.0 ± 6.3) kJ mol−1, in order to estimate the mean (N–O) bond dissociation enthalpy in the gas-phase of 2-amino-3-quinoxalinecarbonitrile-1,4-dioxide. The result obtained is (248.3 ± 8.3) kJ mol−1, which is in excellent agreement with the B3LYP/6-311+G(2d,2p)//B3LYP/6-31G(d) computed value.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...