Carbon–carbon bond formation by radical addition–fragmentation reactions of O-alkylated enols
Abstract
α-tert-Butoxystyrene [H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(OBut)Ph] reacts with α-bromocarbonyl or α-bromosulfonyl compounds [R1R2C(Br)EWG; EWG =
–C(O)X or –S(O2)X] to bring about replacement of the bromine atom by the phenacyl group and give R1R2C(EWG)CH2C(O)Ph. These reactions take place in refluxing benzene or cyclohexane with dilauroyl peroxide or azobis(isobutyronitrile) as initiator and proceed by a radical-chain mechanism that involves addition of the relatively electrophilic radical R1R2(EWG)C˙ to the styrene. This is followed by β-scission of the derived α-tert-butoxybenzylic adduct radical to give But˙, which then abstracts bromine from the organic halide to complete the chain. α-1-Adamantoxystyrene reacts similarly with R1R2C(Br)EWG, at higher temperature in refluxing octane using di-tert-amyl peroxide as initiator, and gives phenacylation products in generally higher yields than are obtained using α-tert-butoxystyrene. Simple iodoalkanes, which afford relatively nucleophilic alkyl radicals, can also be successfully phenacylated using α-1-adamantoxystyrene. O-Alkyl O-(tert-butyldimethylsilyl) ketene acetals H2C
C(OBut)Ph] reacts with α-bromocarbonyl or α-bromosulfonyl compounds [R1R2C(Br)EWG; EWG =
–C(O)X or –S(O2)X] to bring about replacement of the bromine atom by the phenacyl group and give R1R2C(EWG)CH2C(O)Ph. These reactions take place in refluxing benzene or cyclohexane with dilauroyl peroxide or azobis(isobutyronitrile) as initiator and proceed by a radical-chain mechanism that involves addition of the relatively electrophilic radical R1R2(EWG)C˙ to the styrene. This is followed by β-scission of the derived α-tert-butoxybenzylic adduct radical to give But˙, which then abstracts bromine from the organic halide to complete the chain. α-1-Adamantoxystyrene reacts similarly with R1R2C(Br)EWG, at higher temperature in refluxing octane using di-tert-amyl peroxide as initiator, and gives phenacylation products in generally higher yields than are obtained using α-tert-butoxystyrene. Simple iodoalkanes, which afford relatively nucleophilic alkyl radicals, can also be successfully phenacylated using α-1-adamantoxystyrene. O-Alkyl O-(tert-butyldimethylsilyl) ketene acetals H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(OR)OTBS, in which R is a secondary or tertiary alkyl group, react in an analogous fashion with organic halides of the type R1R2C(Br)EWG to give the carboxymethylation products R1R2C(EWG)CH2CO2Me, after conversion of the first-formed silyl ester to the corresponding methyl ester. The silyl ketene acetals also undergo radical-chain reactions with electron-poor alkenes to bring about alkylation–carboxymethylation of the latter. For example, phenyl vinyl sulfone reacts with H2C
C(OR)OTBS, in which R is a secondary or tertiary alkyl group, react in an analogous fashion with organic halides of the type R1R2C(Br)EWG to give the carboxymethylation products R1R2C(EWG)CH2CO2Me, after conversion of the first-formed silyl ester to the corresponding methyl ester. The silyl ketene acetals also undergo radical-chain reactions with electron-poor alkenes to bring about alkylation–carboxymethylation of the latter. For example, phenyl vinyl sulfone reacts with H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(OBut)OTBS to afford ButCH2CH(SO2Ph)CH2CO2Me via an initial silyl ester. In a more complex chain reaction, involving rapid ring opening of the cyclopropyldimethylcarbinyl radical, the ketene acetal H2C
C(OBut)OTBS to afford ButCH2CH(SO2Ph)CH2CO2Me via an initial silyl ester. In a more complex chain reaction, involving rapid ring opening of the cyclopropyldimethylcarbinyl radical, the ketene acetal H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(OCMe2C3H5-cyclo)OTBS reacts with two molecules of N-methyl- or N-phenyl-maleimide to bring about [3 + 2] annulation of one molecule of the maleimide, and then to link the bicyclic moiety thus formed to the second molecule of the maleimide via an alkylation–carboxymethylation reaction.
C(OCMe2C3H5-cyclo)OTBS reacts with two molecules of N-methyl- or N-phenyl-maleimide to bring about [3 + 2] annulation of one molecule of the maleimide, and then to link the bicyclic moiety thus formed to the second molecule of the maleimide via an alkylation–carboxymethylation reaction.
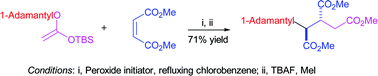

 Please wait while we load your content...
Please wait while we load your content...