Squalene–hopene cyclase: insight into the role of the methyl group on the squalene backbone upon the polycyclization cascade. Enzymatic cyclization products of squalene analogs lacking a 26-methyl group and possessing a methyl group at C(7) or C(11)†
Abstract
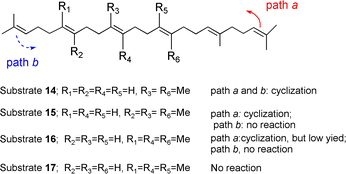
To provide deep insight into the polycyclization reaction of

 Please wait while we load your content...
Please wait while we load your content...