The energetics of isomeric benzoxazine diones: isatoic anhydride revisited
Abstract
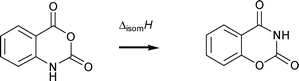
The standard (p° = 0.1 MPa) molar enthalpy of formation of crystalline 2H-1,3-benzoxazine-2,4(3H)dione was measured, at T = 298.15 K, by static bomb calorimetry and the standard molar enthalpy of sublimation, at T = 298.15 K, was obtained using Calvet microcalorimetry. These values were used to derive the standard molar enthalpy of formation in the gaseous phase, T = 298.15 K, of −(401.0 ± 3.5) kJ mol−1. The standard molar enthalpy of sublimation of isatoic anhydride was recalculated, and our recommended experimental value for the standard molar enthalpy of formation in the gaseous phase, T = 298.15 K, is −(406.2 ± 3.4) kJ mol−1. Density functional calculations for the two isomers 2H-1,3-benzoxazine-2,4(3H)dione and isatoic anhydride, in which the ring nitrogen and oxygen have been transposed, confirm the experimental evidence of nearly identical thermochemical stability for these isomers.

 Please wait while we load your content...
Please wait while we load your content...