Chemoselective thioacetalisation and transthioacetalisation of carbonyl compounds catalysed by tetrabutylammonium tribromide (TBATB)†
Abstract
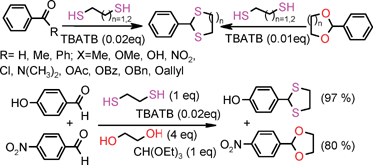
Thioacetals and thioketals of various aldehydes and ketones were obtained directly from carbonyl compounds or by a transthioacetalisation process from cyclic O,O-acetals in the presence of dithiols and a catalytic amount of tetrabutylammonium tribromide (TBATB). Chemoselective thioacetalisation of aromatic aldehydes containing an electron-donating group in the presence of an aldehyde containing an electron-withdrawing group, aldehydes in the presence of ketones, aliphatic cyclic ketones in the presence of aromatic ketones and less hindered ketones in the presence of more hindered ketones have been achieved. A cyclic acetal containing an electron-donating group has been chemoselectively transthioacetalised in the presence of an acetal having an electron-withdrawing substituent. These selectivities are due to the intrinsic reactivity of the substrate themselves and are independent of the catalyst and reaction conditions. Shorter reaction times, mild reaction conditions, stability of acid sensitive protecting groups, high efficiencies, facile isolation of the desired products and the catalytic nature of the reagent are the attractive features of the present method.

 Please wait while we load your content...
Please wait while we load your content...