Synthesis of diphenylcarbazoles as cytotoxic DNA binding agents
Abstract
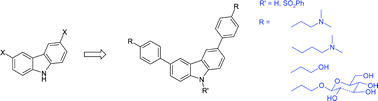
We report the synthesis of a series of novel diphenylcarbazoles designed to interact with DNA. The compounds bearing two or three dimethylaminoalkyloxy side chains were found to bind much more tightly to DNA, preferentially at AT-rich sites, than the corresponding

 Please wait while we load your content...
Please wait while we load your content...