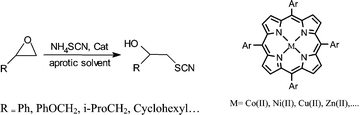
Metalloporphyrins as new catalysts in the mild, efficient and regioselective conversion of epoxides to β-hydroxy thiocyanates with NH4SCN
Abstract
The regioselective cleavage of 1,2-epoxyethanes to 2-hydroxyethyl thiocyanates with ammonium thiocyanates in the presence of some

 Please wait while we load your content...
Please wait while we load your content...