Isomers of dichlorobis(2-phenylazopyridine)ruthenium(II)
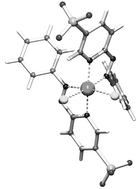
[Ru(azpy)2Cl2], especially the so-called α isomer, display remarkably high cytotoxicities against human tumor cell lines. Unfortunately, the parent [Ru(azpy)2Cl2] compounds are poorly water-soluble. In this paper the synthesis and characterization of the new water-soluble ligand 2-phenylazopyridine-5-sulfonic acid (Hsazpy) is described. Use of this ligand in reaction with RuCl3 gave two isomers, which were isolated as α- and β-[NEt4]2[Ru(sazpy)2Cl2]. The compounds have been fully characterized by (2D) NMR spectroscopy. The molecular structure of the α isomer of [NEt4]2[Ru(sazpy)2Cl2]·2H2O has been determined by single-crystal structure analysis. The packing in the crystal structure of α-[NEt4]2[Ru(sazpy)2Cl2]·2H2O shows an interesting hydrogen-bonding pattern in which two water molecules are involved. One water molecule bridges between a Cl ligand and a SO3− group within one ruthenium moiety, the other water molecule forms a bridge between two SO3− groups from two different ruthenium centers, resulting in a chain-like structure. Preliminary evaluation of the cytotoxicity by means of the IC50 value in A2780 cell line classifies α-[NEt4]2[Ru(sazpy)2Cl2] as non-toxic, but this does not rule out other anticancer activities.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...