Rectifying Au–S–CnH2n–P3CNQ derivatives
Abstract
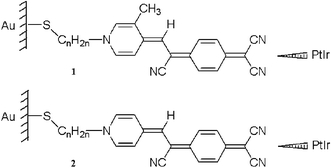
Self-assembled monolayers (SAMs) obtained from Z-β-[N-(ω-acetylsulfanylalkyl)-3-methyl-4-pyridinium]-α-cyano-4-styryldicyanomethanide (1), a pyridinium analogue of the extensively studied rectifying dyes, C16H33–Q3CNQ (T. Xu, I. R. Peterson, M. V. Lakshikantham and R. M. Metzger, Angew. Chem., Int. Ed. Engl., 2001, 40, 1749; N. Okazaki, J. R. Sambles, M. J. Jory and G. J. Ashwell, Appl. Phys. Lett., 2002, 81, 2300 [] and G. J. Ashwell, A. Chwialkowska and L. R. H. High, J. Mater. Chem., 2004, 14, 2389 []) and Au–S–CnH2n–Q3CNQ ( and G. J. Ashwell, R. Hamilton and L. R. H. High, J. Mater. Chem., 2003, 13, 1501 []), exhibit asymmetric current-voltage (I–V) characteristics. Protonation of the C(CN)2 group disrupts the molecule's donor–acceptor structure and the rectification is switched off and on by exposure to acid and base respectively. Molecular dimensions within the films are consistent with the chromophores being closely packed and upright: the cross-sectional area is 0.29 ± 0.03 nm2 molecule−1 and the monolayer thickness increases from 1.7 ± 0.1 to 2.2 ± 0.1 nm as the linking group is lengthened from C3H6 to C10H20. The different insulating links have no significant effect on the electrical asymmetry, the rectifying behavior being attributed to the donor–(π-bridge)–acceptor moiety, which is sterically hindered and non-planar. This inhibits through-molecule orbital overlap of the electroactive end groups. Rectification is suppressed in SAMs obtained from Z-β-[N-(ω-alkylsulfanylalkyl)-4-pyridinium]-α-cyano-4-styryldicyanomethanide (2) where the D–π–A moiety is rendered less sterically hindered by eliminating the 3-methyl group of 1.

 Please wait while we load your content...
Please wait while we load your content...