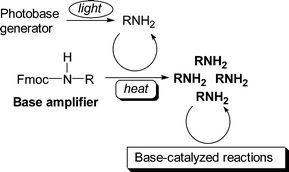
A novel concept of base proliferation for improving the photosensitivity of base-sensitive materials is described by presenting the autocatalytic transformation of 9-fluorenylmethyl carbamates to aliphatic amines. A 9-fluorenylmethyl carbamate, as a base amplifier, was subjected to a base-catalysed fragmentation reaction to liberate the corresponding amine, which can then act as a catalyst for decomposing parent molecules, leading to autocatalytic decomposition. Consequently, the amine is generated from an equimolar amount of the carbamate using a catalytic amount of the same amine. 1-(9-Fluorenylmethoxycarbonyl)piperidine and 1-(9-fluorenylmethoxycarbonyl)cyclohexylamine were suitable as base amplifiers because of their thermal stability under neutral conditions and high base-catalytic reactivity. On the basis of the results, 1,3-bis[1-(9-fluorenylmethoxycarbonyl)-4-piperidyl]propane and 1,6-bis[(9-fluorenylmethoxy)carbonylamino]hexane were designed as base amplifiers which liberate aliphatic diamines to crosslink poly(glycidyl methacrylate) photochemically in the presence of a photobase generator. Addition of the base amplifiers resulted in a marked improvement of the photosensitivity characteristics of the polymer by a factor of 16 and 50, respectively.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...