Green chemistry analytical method development: a revisit on the use of potassium ferrioxalate as a chemical actinometer
Abstract
The experimental details regarding the use of potassium ferrioxalate (3) as a chemical actinometer are discussed with respect to the precision, ruggedness and simplicity of the method. Despite the fact that as much as a ten-fold difference between the actinometer 3 quantum efficiency and the analyte quantum efficiency was observed, these differences were compensated for by the use of multiple actinometer solutions during the quantum yield determinations. As a tool in process development, the advantages of the merry-go-round apparatus for the evaluation of the influence of experimental conditions for the quantum yield of photochemical reactions are also discussed.
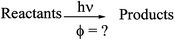

 Please wait while we load your content...
Please wait while we load your content...