Ene reaction of allylbenzene and N-methylmaleimide in subcritical water and ethanol
Abstract
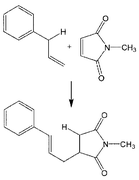
Ene reaction of allylbenzene and N-methylmaleimide was studied in water and ethanol solvents at subcritical temperatures (220–310 °C). Subcritical water was inappropriate for this reaction, because it rapidly hydrolyzed N-methylmaleimide. Subcritical ethanol was found to be a very promising solvent. The highest ene product yield in ethanol reached 40% in 480 min, and the highest trans-selectivity was 92%. The yields in pure ethanol were comparable to those in 1,2,4-trichlorobenzene with 10% hydroquinone added as a polymerization inhibitor. Addition of hydroquinone had a negligible effect on the yield in ethanol, suggesting that the solvent ethanol itself acts as an inhibitor of the side reactions. It is also expected that the polar environment and the high vapor pressure of ethanol favored pericyclic association between the apolar starting compounds.

 Please wait while we load your content...
Please wait while we load your content...