New mixed alkaline-earth nitridomolybdate(vi) nitride oxides with anion-ordered sub-structures†
Abstract
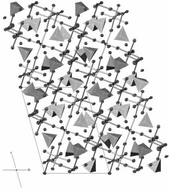
New molybdenum(VI) nitride ![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (no. 2). The mixed alkaline earth compounds with composition Ca38Sr13[MoN4]12N8O3 and Ca36Sr15[MoN4]12N8O3 are isostructural with the quaternary nitride oxides Sr51[WN4]12N8O3 and Ca51[WN4]12N8O3. The structures contain isolated [MoN4]6− tetrahedra, partially disordered
(no. 2). The mixed alkaline earth compounds with composition Ca38Sr13[MoN4]12N8O3 and Ca36Sr15[MoN4]12N8O3 are isostructural with the quaternary nitride oxides Sr51[WN4]12N8O3 and Ca51[WN4]12N8O3. The structures contain isolated [MoN4]6− tetrahedra, partially disordered

 Please wait while we load your content...
Please wait while we load your content...