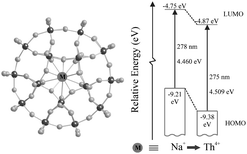
The ground state electronic properties of metal-exchanged Preyssler heteropolyoxoanions [Mn+P5W30O110]n−15, in which the encapsulated Mn+ ions are the spherical, diamagnetic ions Na+, Ca2+, Sr2+, Y3+, La3+ and Th4+, are studied using a combination of electrochemical, optical, and NMR experiments. We have designed experiments that focus on the influence of the charge (n) of the encapsulated cations, which themselves have no redox response, and its effect upon the W–O framework MOs. As n increases, the cluster anions accept electrons into their LUMOs with increasing ease, and their lowest-energy LMCT bands reveal a corresponding blue shift, which is indicative of an increase of the LUMO–HOMO energy splitting with increasing n. 183W NMR spectra are used to identify the atomic origin of the LUMO states, which are shown to be composed primarily of orbitals from the ring of 5 W atoms near Mn+. The cation charge correlates directly and linearly with the half-wave potentials of the first redox couples, the LMCT band energies, and the W chemical shifts. We have combined this suite of experimental results to construct an energy level diagram of the frontier MOs for the Preyssler cluster anions. In so doing, we provide a fundamental perspective that is not otherwise available on the cation's role with specific regard to the electronic behavior of the W–O orbitals. These results are expected to provide benchmarking information as theorists begin to study these large POM systems.

 Please wait while we load your content...
Please wait while we load your content...