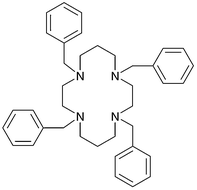
Host–guest formation between 1,4,8,11-tetrabenzyl-1,4,8,11-tetraazacyclodecane (1) and lipophilic organic carboxylic acids in chloroform has been investigated and the effect of such ligand assembly on the solvent extraction of copper(II) and silver(I) has been probed. NMR titration experiments in the absence of a metal ion confirm the formation of weak 1 ∶ 1 and 1 ∶ 2 (macrocycle ∶ carboxylic acid) assemblies in CDCl3 between 1 and palmitic (hexadecanoic) acid or 4-tert-butylbenzoic acid while difunctional salicylic acid showed a 1 ∶ 2 interaction that is somewhat stronger. The interaction between the former two acids and the tetra-N-benzylated macrocycle is significantly less than that reported previously for its non-substituted parent, cyclam; a result that likely reflects the presence of the less-basic, more sterically hindered tertiary nitrogens in 1 relative to the secondary nitrogens present in cyclam. Carboxylic acid-containing assemblies of this type have been used as extractants in a series of solvent extraction (water/chloroform) experiments. From both previous observations as well as from entropy considerations, it was anticipated that the use of a host–guest assembly of the above type for metal-ion complexation might contribute to enhanced metal ion binding (and concomitant enhanced metal ion extraction). Such behaviour is postulated to arise from the components of the coordination sphere being, at least in part, assembled for complex formation. In accord with this, the use of the ligand assembly involving palmitic acid/macrocycle 1 was found to lead to enhanced (synergistic) extraction of copper(II) at a metal ion concentration of 10−3 mol dm−3 while, for silver(I), synergism was somewhat marginal at this concentration but was clearly apparent under related conditions when the silver concentration was reduced to 10−4 mol dm−3. Similar behaviour towards silver was also observed when 4-tert-butylbenzoic acid was substituted for palmitic acid, while the use of salicylic acid resulted in enhanced (synergistic) extraction at both metal ion concentrations.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...