Cyclometallated palladium(II) azido complexes containing C,N,N- or C,N-donor ligands, [Pd(N3)L]
(HL = 6-phenyl-2,2′-bipyridine or 2-phenylpyridyl derivatives), showed different reactivities toward organic isocyanides and isothiocyanates. In particular, aryl isocyanides (CN–Ar) underwent insertion into the orthometallated Pd–C bond on the phenyl moiety of the supporting ligand (L) in [Pd(N3)L] or [Pd(N3)(PR3)L] to selectively give carbodiimido {[Pd(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–Ar)L]}, imidoyl {[Pd(N3)(–C
N–Ar)L]}, imidoyl {[Pd(N3)(–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–Ar)(PR3)L]}, or imidoyl carbodiimido complexes {[Pd(N
N–Ar)(PR3)L]}, or imidoyl carbodiimido complexes {[Pd(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–Ar)(–C
N–Ar)(–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–Ar)L] or [Pd(N
N–Ar)L] or [Pd(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–Ar)(–C
N–Ar)(–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–Ar)(PR3)L]}, depending on reaction conditions. Interestingly, reactions of [Pd(N3)(PR3)L] with organic isothiocyanates gave unusual dinuclear complexes [(μ-SCN4–R)PdL]2, exhibiting the concurrent S- and N-coordinating thio–tetrazole bridge.
N–Ar)(PR3)L]}, depending on reaction conditions. Interestingly, reactions of [Pd(N3)(PR3)L] with organic isothiocyanates gave unusual dinuclear complexes [(μ-SCN4–R)PdL]2, exhibiting the concurrent S- and N-coordinating thio–tetrazole bridge.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–Ar)L]}, imidoyl {[Pd(N3)(–C
N–Ar)L]}, imidoyl {[Pd(N3)(–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–Ar)(PR3)L]}, or imidoyl carbodiimido complexes {[Pd(N
N–Ar)(PR3)L]}, or imidoyl carbodiimido complexes {[Pd(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–Ar)(–C
N–Ar)(–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–Ar)L] or [Pd(N
N–Ar)L] or [Pd(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–Ar)(–C
N–Ar)(–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–Ar)(PR3)L]}, depending on reaction conditions. Interestingly, reactions of [Pd(N3)(PR3)L] with organic
N–Ar)(PR3)L]}, depending on reaction conditions. Interestingly, reactions of [Pd(N3)(PR3)L] with organic 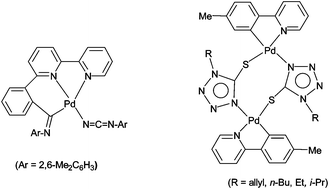

 Please wait while we load your content...
Please wait while we load your content...