The tris(oxalato)cobaltate(III) complex [Co(C2O4)3]3−, EoCoIII/II
=
+0.57 V) is readily reduced by the 2e− reagents, Sn(II) and Ge(II), in contrast to (NH3)5CoCl2+ and (NH3)5CoBr2+, which are unreactive toward these donors. Rates for the oxalato oxidant are only 10−3–10−2 as great as those for vitamin B12a
(aquacob(III)alamin, Eo
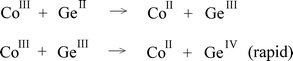
+0.35 V at pH 1), in accord with the suggestion that reductions of corrin-bound cobalt(III) by Sn(II) and Ge(II) occur predominantly through an additional path involving Co(I). Reductions of the oxalato complex by 2e− donors are taken to proceed by initial formation of odd-electron intermediates (e.g., SnIII and GeIII) which react rapidly with CoIII. Such a two-step sequence is in keeping with the observed behavior of the rare reductant, TiII, which is found to be oxidized by [Co(C2O4)3]3− more slowly than (independently prepared) Ti(III) under comparable conditions.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...