Molecular, supramolecular and solution structures of peroxovanadium complexes with ON and O3N donor set ligands: two new types of cationic–anionic peroxovanadium(v) peroxovanadates(v)
Abstract
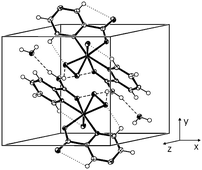
The complexes, [VO(O2)(pa)2]ClO4·3H2O (1), [VO(O2)(pa)2][VO(O2)2(pa)]·3H2O (2), [VO(O2)(pa)2][VO(O2)(ada)]·2H2O (3) and [VO(O2)(pa)(pca)]·H2O (4)
[pa = picolinamide, ada = carbamoylmethyliminodiacetate(2−) and pca = 2-pyrazinecarboxylate(1−)], were synthesized. 2 and 3 are new types of peroxovanadium complexes: monoperoxovanadium diperoxovanadate (2) and monoperoxovanadium monoperoxovanadate (3). The complexes were characterized by chemical analysis and IR spectroscopy, and 1, 3 and 4 also by X-ray analysis. The structure of 1 is disordered, with alternating positions of the oxo and peroxo ligands. The peroxo oxygen atoms, Op, in 1 are involved in weak hydrogen bonds with water molecules and close intramolecular C–H⋯Op bonds [d(H⋯Op)
∼ 2.0 Å]. The supramolecular structure of 1 is formed by a network of hydrogen bonds and strong attractive intermolecular π–π interactions between the pyridine rings. The supramolecular architecture in 4 is constructed by (N,O)–H⋯O hydrogen bonds between the neutral complex molecules and water of crystallization. The peroxo oxygen atoms in 4 form intramolecular C–H⋯Op bonds [d(H⋯Op)
= 2.303 Å]. The pa and pca ligands are ON coordinated via the oxygen atoms of the C(NH2)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and COO− groups, respectively, and nitrogen atoms of the heterocyclic rings, and ada as a tetradentate O3N ligand. The thermal analysis of 4 showed that the loss of water of crystallization and the active oxygen release (Tmin/°C 82, Tmax/°C 165) are, under given conditions, individual processes separated by the temperature interval 90–132 °C. The solution structures and stability were studied by UV-VIS and 51V NMR spectroscopies.
O and COO− groups, respectively, and nitrogen atoms of the heterocyclic rings, and ada as a tetradentate O3N ligand. The thermal analysis of 4 showed that the loss of water of crystallization and the active oxygen release (Tmin/°C 82, Tmax/°C 165) are, under given conditions, individual processes separated by the temperature interval 90–132 °C. The solution structures and stability were studied by UV-VIS and 51V NMR spectroscopies.

 Please wait while we load your content...
Please wait while we load your content...