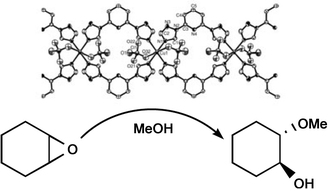
The structures of new polymeric compounds containing Cu(II) ions and btp (2,6-bis(N′-1,2,4-triazolyl)pyridine) ligands have been determined. The btp ligands bridge Cu(II) ions to form double zigzag chains, [Cu(ClO4)2(btp)2]
3 with perchlorate anions, and form single zigzag chains, [Cu(btp)(H2O)4](SO4)·2H2O 4 with sulfate anions. The polymeric compound 3 was found to effectively catalyze the epoxide ring-opening reaction with methanol, while polymeric compound 4 was almost inactive with epoxides under the same conditions. The polymeric compound 3 showed an efficient catalytic activity and regioselective reactivity in the ring opening of epoxides and allowed reuse without a significant loss of activity through three runs with epoxides.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...