Substrate binding to vanadate-dependent bromoperoxidase from Ascophyllum nodosum: A vanadium K-edge XAS approach†
Abstract
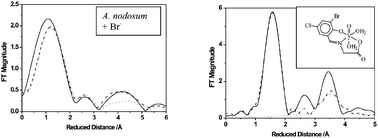
The EXAFS region of vanadium K-edge XAS spectra of native vanadate-dependent bromoperoxidase (isoenzyme I) from Ascophyllum nodosum in the presence of the substrate bromide can be fitted to three shells (at 1.62, 1.73–1.78 and 1.99–2.07 Å) in the first coordination sphere of vanadium plus two more distant shells at 4.1 Å, possibly corresponding to bromide, and 4.7 Å due to light scatterers stemming from the protein pocket. Bromide does not directly bind to the vanadium centre. The XANES and the EXAFS features for the enzyme are essentially reproduced by model complexes of the general composition [VO(H2O)n(ONO)] (n = 1 or 2) where ONO is the dianion of a Schiff base from bromosalicylaldehydes (Brsal; with the Br substituent in the position 3, 4, 5 or 6) and amino acids. The 3-Brsal derivatives exhibit an outer sphere shell at 3.8 Å, which is traced back to intermolecular contacts. The data obtained from EXAFS are compared to those obtained from single crystal X-ray diffraction of [VO(H2O)2(4-Brsal-gly)] and [VO(H2O)2(6-Brsal-gly)] (gly = glycinate). In the complex [VOBr2(ONO)′] ((ONO)′ is the Schiff base from o-anisole and o-hydroxyaniline), the V–Br distance is 2.44 Å.

 Please wait while we load your content...
Please wait while we load your content...