Synthesis, crystal structure and hydroformylation activity of triphenylphosphite modified cobalt catalysts
Abstract
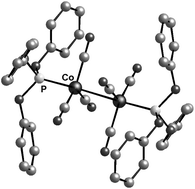
The dinuclear complex [Co2(CO)6{P(OPh)3}2] (2) has been synthesised and was fully characterised. The solid state structure revealed a trans diaxial geometry, no bridging carbonyls, and Co–Co and Co–P bond lengths of 2.6722(4) and 2.1224(4) Å, respectively. Catalysed hydroformylation of 1-pentene with 2 was attempted at temperatures in the range 120 to 210 °C and pressures between 34 and 80 bar. High pressure spectroscopy (HP-IR and HP-NMR) was used to detect hydride intermediates. High pressure infrared (HP-IR) studies revealed the formation of [HCo(CO)3P(OPh)3] (4) at ca. 110 °C, but at higher temperatures absorption bands corresponding to [HCo(CO)4] (3) were observed. The hydride intermediate 4 has also been synthesised and characterised. Upon increased ligand concentration, HP-IR studies showed the formation of new carbonyl absorption bands due to a higher substituted cobalt carbonyl complex-[HCo(CO)2{P(OPh)3}2] (5), which is believed to be catalytically less active. Complex 5 has been synthesised independently and was fully characterised. A low temperature crystal structural study of 5 revealed a trigonal bipyramidal structure with a trans H–Co–CO arrangement and two equatorial phosphite ligands, the Co–P bond lengths being 2.1093(8) and 2.1076(8) Å, respectively.

 Please wait while we load your content...
Please wait while we load your content...