Models for biological trinuclear copper clusters. Characterization and enantioselective catalytic oxidation of catechols by the copper(ii) complexes of a chiral ligand derived from (S)-(−)-1,1′-binaphthyl-2,2′-diamine†
Abstract
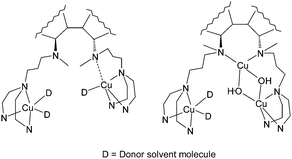
The dinuclear and trinuclear Cu(II) complexes of an octadentate ligand derived from (S)-1,1′-binaphthyl-2,2′-diamine have been prepared and characterized by UV/Vis, CD, EPR and NMR spectroscopy. The ligand contains two tridentate aminobis(benzimidazole) donor arms connected to a central bidentate diaminobinaphthyl linker, which hosts the chiral unit. In the dinuclear Cu complex the ligation occurs essentially within the tridentate arms of the ligand. The two Cu centers are EPR nonequivalent and noninteracting. The EPR data suggests that one of the Cu ions additionally interacts with one of the tertiary aminonaphthyl donors. In the trinuclear complex the two aminonaphthyl donors bind the third Cu ion. The EPR spectrum of this complex shows the signal for a mononuclear Cu(II) center bound to a tridentate arm, while the remaining two Cu(II) centers are coupled through hydroxo groups. The CD spectrum shows that in the free ligand a severe reduction of the dihedral angle between the naphthyl groups from the strain free range occurs. This conformation is stabilized by ring stacking interactions with the benzimidazole groups. On complex formation this interaction is removed because the benzimidazole groups are involved in metal binding. In the dinuclear Cu complex the conformation of the binaphthyl chromophore probably approaches the strain free range, while in the trinuclear Cu complex a marked flattening of the dihedral angle between the two naphthyl rings occurs. Both complexes are active catalysts in the oxidation of L-/D-Dopa derivatives to quinones. High enantioselectivity is observed in the oxidation of L-/D-Dopa methyl ester catalyzed by the dinuclear Cu complex, which exhibits strong preference for the D enantiomer. The enantioselectivity is largely lost for the trinuclear Cu complex.

 Please wait while we load your content...
Please wait while we load your content...