Aminoacid N-substituted 1,4,7-triazacyclononane and 1,4,7,10-tetraazacyclododecane Zn2+, Cd2+ and Cu2+ complexes. A preparative, potentiometric titration and NMR spectroscopic study†
Abstract
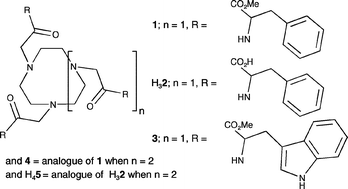
The pKas and Zn2+, Cd2+ and Cu2+ complexation constants (K) for 1,4,7-tris[(2″S)-acetamido-2″-(methyl-3″-phenylpropionate)]-1,4,7-triazacyclononane, 1, 1,4,7-tris[(2″S)-acetamido-2″-(1″-carboxy-3″-phenylpropane)]-1,4,7-triazacyclononane, H32, 1,4,7-tris[(2″S)-acetamido-2″-(methyl-3″-(1H-3-indolyl)propionate)]-1,4,7-triazacyclononane, 3, and 1,4,7,10-tetrakis[(2″S)-acetamido-2″-(methyl-3″-phenylpropionate)]-1,4,7,10-tetraazacyclododecane, 4, 1,4,7,10-tetrakis[(2″S)-acetamido-2″-(1″-carboxy-3″-phenylpropane)]-1,4,7,10-tetraazacyclododecane, H45, in 20 ∶ 80 v/v water–methanol solution are reported. The pKas within the potentiometric detection range for H313+ = 8.69 and 3.59, for H623+ = 9.06, 6.13, 4.93 and 4.52, H333+ = 8.79 and 3.67, H444+ = 8.50, 5.62 and 3.77 and for H854+ = 9.89, 7.06, 5.53, 5.46, 4.44 and 4.26 where each tertiary amine nitrogen is protonated. The complexes of 1: [Zn(1)]2+ ( 9.00), [Cd(1)]2+ (6.49), [Cd(H1)]3+ (4.54) and [Cu(1)]2+ (10.01) are characterized by the log(K/dm3 mol−1) values shown in parentheses. Analogous complexes are formed by 3 and 4: [Zn(3)]2+ (10.19), [Cd(3)]2+ (8.54), [Cu(3)]2+ (10.77), [Zn(4)]2+ (11.41) [Cd(4)]2+ (9.16), [Cd(H4)]3+ (6.16) and [Cu(4)]2+ (11.71). The tricarboxylic acid H32 generates a greater variety of complexes as exemplified by: [Zn(2)−] (10.68) [Zn(H2)] (6.60) [Zn(H22)+] (5.15), [Cd(2)]− (4.99), [Cd(H2)] (4.64), [Cd(H22)]+ (3.99), [Cd(H32)]2+ (3.55), [Cu(2)]− (12.55) [Cu(H2)] (7.66), [Cu(H22)]+ (5.54) and [Cu2(2)]4− (3.23). The complexes of H45 were insufficiently soluble to study in this way. The 1H and 13C NMR spectra of the ligands are consistent with formation of a predominant Zn2+ and Cd2+ Δ or Λ diastereomer. The preparations of the new pendant arm macrocycles H32, 3, 4 and H45 are reported.

 Please wait while we load your content...
Please wait while we load your content...