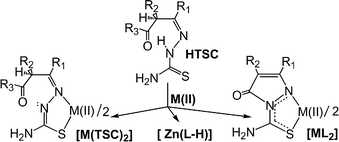
Zinc(II) and cadmium(II) acetates were reacted in methanol under various experimental conditions with thiosemicarbazones derived from β-keto amides or β-keto esters (HTSC). Some of these reactions afforded thiosemicarbazonate complexes [M(TSC)2] with IR and NMR spectra compatible with N,S-coordination, but most gave complexes [ML2], where HL is a substituted 2,5-dihydro-5-oxo-1H-pyrazole-1-carbothioamide resulting from cyclization of the HTSC. Some of these pyrazolonates and two of the HL ligands were studied by X-ray diffractometry, and their structures are discussed. Surprisingly, the reactions of zinc(II) acetate with HTSC in 1 ∶ 1 mol ratio usually gave a third, previously unreported type of complex with a dideprotonated ligand, [Zn(L − H)], which was also formed when [ZnL2] and Zn(OAc)2 interacted at room temperature in 1 ∶ 1 mol ratio. These L − H complexes are highly insoluble in all common solvents, which hinders their characterization but suggests that they are polymeric in nature.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...