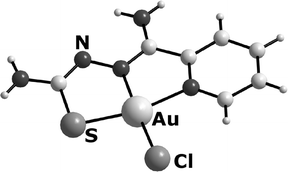
Novel thiosemicarbazonato complexes of gold(III) have been prepared from reactions of [Au(damp-C1,N)Cl2
(damp- = 2-(N,N-dimethylaminomethyl)phenyl) or [NBu4][AuCl4] with 2-pyridineformamide thiosemicarbazones (HL). The thiosemicarbazones deprotonate and coordinate as mononegative, tridentate NNS ligands to gold to give [Au(Hdamp-C1)(L)]Cl2 or [AuCl(L)]Cl complexes. The organometallic damp− ligand is protonated during the reactions and the Au–N bond is cleaved. The [AuCl(L)]+ cations represent the first gold(III) complexes with thiourea derivatives which are not stabilised by an additional organometallic ligand. Reactions of [NBu4][AuX4]
(X = Cl, Br) with diphenylthiocarbazone (dithizone) result in reduction of the metal and the formation of gold(I) complexes of the composition [AuX(SCN4-3,4-Ph2)] where SCN4-3,4-Ph2 is 3,4-diphenyltetrazole thione which is formed from cyclisation of dithizone.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...