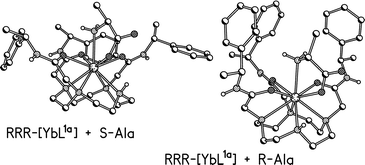
A detailed investigation of the nature of the binding of each of the 20 common α-amino acids and various selected dipeptides to a chiral, diaqua–ytterbium complex in aqueous solution has been carried out. Analysis of the dipolar 1H NMR paramagnetic shifts suggests that the α-amino acids form a common chelated structure within a nine-coordinate mono-capped square antiprismatic coordination environment, with the amine N axially disposed. Crystal structures of nine chelated YbL1–amino acid adducts (Gly, Ala, Ser, Thr, Met) confirm this. The ternary complexes with dipeptides (e.g. Gly-Ala, Gly-Ser, Gly-Met, Gly-Asp, Gly-Asn, Gly-His, Ser-Met, Asp-Phe, His-Gly) also favour the terminal amine as the axial donor with the proximate amide group binding to generate a five-ring chelate. Evidence for chelation through side-chain functionality was found only in the case of N-terminal Asp. The chiral environment about the ytterbium ion upon amino acid binding has also been probed using near-IR circular dichroism spectroscopy.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...