Novel oxygen storage mechanism based on redox of sulfur in lanthanum oxysulfate/oxysulfide
Abstract
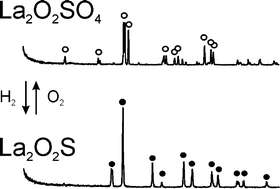
A sulfur redox cycle between La2O2SO4(S6+) and La2O2S(S2−) phases was found for the first time to achieve the oxygen storage of 2 mol O2 mol−1, which is eight times larger than that of the conventional CeO2–ZrO2 system.

 Please wait while we load your content...
Please wait while we load your content...