The kinetics of the chemical and physical quenching of the first excited singlet state of oxygen [1O2
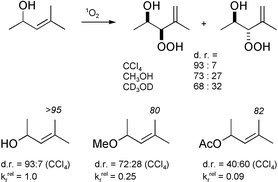
(1Δg)] by unfunctionalized alkenes 1–4, allylic alcohols 5–7 and 9, allylic acetates 8 and 11, and the allylic ether 10 display small solvent-polarity effects on the reactivity. The regioselectivity of the singlet oxygen ene reaction is solvent independent for the unfunctionalized alkenes as well as the prenol-type substrates, the latter showing substantial solvent effects on the diastereoselectivity. Pronounced physical quenching is detected only for the allylic alcohols 5 and 6. These results are interpreted in terms of the interactions between singlet oxygen and the allylic hydroxy groups, conformationally promoted by allylic strain which lead either to chemical activation or to physical quenching. The results for substrate 9 in deuterated vs. non-deuterated methanol are in accord with hydrogen bonding between the allylic alcohol and 1O2, which directs the diastereoselectivity of the ene reaction with chiral allylic alcohols.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...