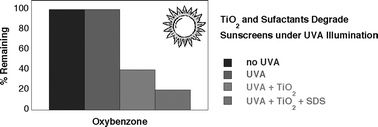
The photostability of the widely used UVB sunscreen agents 2′-ethylhexyl-2-cyano-3-phenylcinnamate (1), 2-hydroxy-4-methoxybenzophenone (2), octyl salicilate (3), and 2′-ethylhexyl-4-methoxycinnamate (4) has been investigated under UVA irradiation in the absence and presence of TiO2, an inorganic filter commonly employed in combination with organic filters in sunscreen preparations. In the absence of TiO2, 1–3 are photostable and 4 undergoes the expected E–Z isomerization; the presence of TiO2 caused mineralization of the organic filters and, surprisingly, the process is noticeably faster in the presence of surfactant than in sunscreen and water suspensions. The results indicate that in water suspension, mineralization is likely to occur on or near the TiO2 particle surface; when the organic sunscreens are segregated in the micelle core, reactive radicals, produced during TiO2-promoted degradation of the micellar system, may participate in sunscreen degradation. In addition, a pre-fluorescent probe for carbon-centered radical detection, 4-(3-hydroxy-2-methyl-4-quinolineoxy)-2,2,6,6,-tetramethylpiperidine-1-oxyl free radical or QT (5), was employed to demonstrate that carbon-centered radicals are evolved during micelle degradation and may participate in the mineralization of sunscreens.

 Please wait while we load your content...
Please wait while we load your content...