Stereochemistry and mechanism of the conversion of 5-aminolaevulinic acid into porphobilinogen catalysed by porphobilinogen synthase†
Abstract
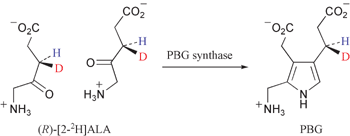
(3R)- and (3S)-Deuteriated forms of 5-aminolaevulinic acid have been synthesised and the (3R)-form shows a significantly larger isotope effect when incubated with

 Please wait while we load your content...
Please wait while we load your content...