Biomimetic total synthesis of forbesione and desoxymorellin utilizing a tandem Claisen/Diels–Alder/Claisen rearrangement†
Abstract
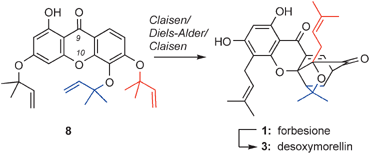
A concise synthesis of forbesione (1) and desoxymorellin (3) is presented. Central to the strategy is a biomimetic Claisen/Diels–Alder/Claisen reaction cascade that proceeds in a regioselective manner and produces the desired scaffold exclusively. The observed regioselectivity and product distribution of the Claisen/Diels–Alder/Claisen reaction are attributed to the electronic effects of the

 Please wait while we load your content...
Please wait while we load your content...